Berkeley, California/berkeley296
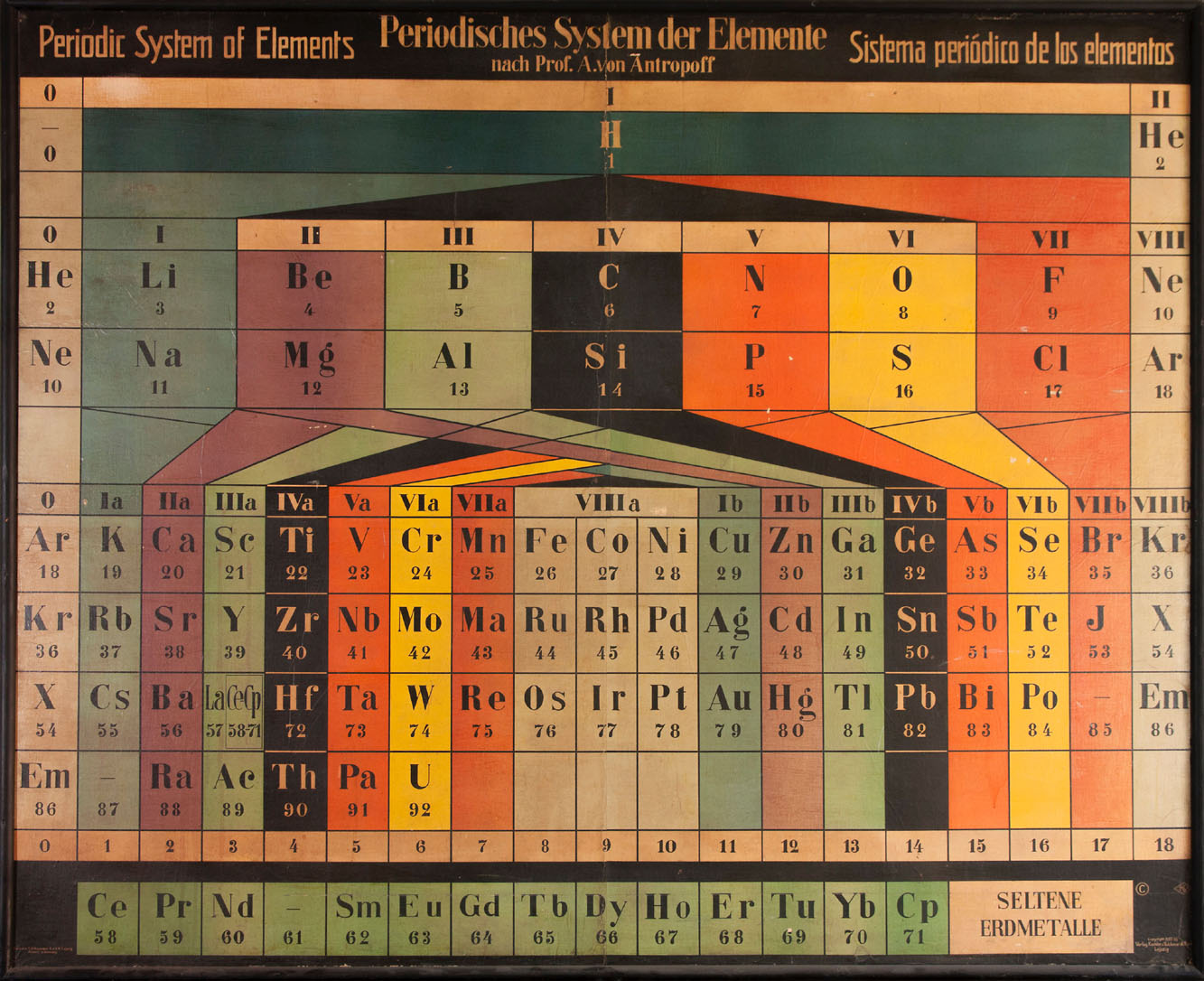
Previous | Home | NextInside Gilman Hall, a copy of the historically intriguing copy of the Antropoff Periodic Table is presented (Andreas von Antropoff (1878-1956, of Baltic-German extraction). This was the most popular Periodic Table in Germany for several years. It descriptively combines the features of a short Periodic Table -- where, for example, Ti and Ge are related, both with a valence of +4, to C and Si -- and a long Periodic Table -- where Ge is more closely related to C and Si, while Ti is a transition metal. Antropoff includes "masurium," element 43 (putatively discovered by Walter and Ida Noddack); and "casseopeium" (discovered by Welsbach who claimed as priority over Urbain's lutetium, resulting in a bitter nationalistic struggle). The rare earths are correctly separated out and not interrelated as in other attempts at the Periodic Table by other chemists. Element number zero (neutronium) is positioned at the very top; both its theoretical and chemical significance was not clear. The elements 90-92 (thorium, protactinium, and uranium) were not yet separated out in a separate row, even though Bohr had proposed their behavior was analogous to the lanthanides (the "actinides" had to await the era of Seaborg) but instead were considered part of the main Periodic Table. Element 86 was not yet called "radium" but instead "Emanation," a term initiated by Ernest Rutherford. "X" is the symbol for xenon; "J" is the symbol for Jod (iodine). The noble gases appear at both ends because the table actually portrays an unwrapped spiral.